Standards Impurities & Metabolites
Pharmaceutical Reference Standards / Green Standards Food
And Beverage Standards / Agro-Chemical Standards / Environmental Standards
https://en.wikipedia.org/wiki/Pharmaceutical_formulation
https://en.wikipedia.org/wiki/Drug_reference_standard
https://www.ich.org/products/guidelines/quality/quality-single/article/impurities-in-new-drug-substances.html
https://en.wikipedia.org/wiki/Metabolomics

Packages of medication
Other names :
medicine, drug, pharmaceutical, pharmaceutical preparation, pharmaceutical
product, medicinal product, medicament, remedy. |
Drug reference
standard
1. Definition
A pharmaceutical reference standard is a highly characterized material suitable
to test the identity, strength, quality and purity of substances for
pharmaceutical use and medicinal products.
2. Pharmacopoeial Reference standards
Pharmacopoeial Reference standards are a subset of Pharmaceutical Reference
Standards. They are established for the intended use described in Pharmacopeial
texts (monographs and general chapters). Pharmacopeial Reference Standards are
available from various pharmacopoeias such as United
States Pharmacopeia and the European
Pharmacopoeia. Where pharmacopoeial tests or assays call for the use of a
Pharmacopoeial Reference Standard, only those results obtained using the
specified Pharmacopoeial Reference Standard are conclusive.
|
Impurities in new drug substances are addressed from two perspectives: Chemistry
Aspects include classification and identification of impurities, report
generation, listing of impurities in specifications, and a brief discussion of
analytical procedures; and Safety Aspects include specific guidance for
qualifying those impurities that were not present, or were present at
substantially lower levels, in batches of a new drug substance used in safety
and clinical studies. |

|
Classification of impurities :
Impurities can be classified into the following categories:
• Organic impurities (process- and drug-related)
• Inorganic impurities
• Residual solvents
Organic impurities can arise during the manufacturing process and/or storage of
the new drug substance. They can be identified or unidentified, volatile or
non-volatile, and include:
• Starting materials
• By-products
• Intermediates
• Degradation products
• Reagents, ligands and catalysts
Inorganic impurities can result from the manufacturing process. They are
normally known and identified and include:
• Reagents, ligands and catalysts
• Heavy metals or other residual metals
• Inorganic salts
• Other materials (e.g., filter aids, charcoal)
|
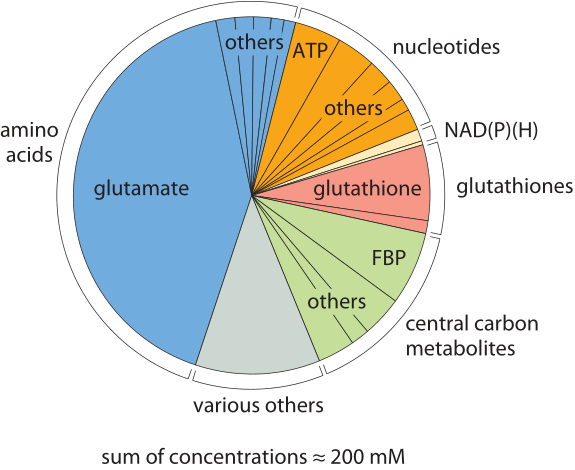
Free metabolites for an E. coli cell growing on glucose.
|
A metabolite is the
intermediate end product of metabolism. The term metabolite is
usually restricted to small molecules. Metabolites have various
functions, including fuel, structure, signaling, stimulatory and inhibitory
effects on enzymes, catalytic activity of their own (usually as a cofactor to an enzyme), defense, and
interactions with other organisms (e.g. pigments, odorants, and pheromones). A primary
metabolite is directly involved in normal "growth", development, and
reproduction. Ethylene is an example of a primary metabolite produced
in large-scale by industrial microbiology. A secondary metabolite is not directly
involved in those processes, but usually has an important ecological function. Examples include antibiotics and pigments such as resins and terpenes etc. Some
antibiotics use primary metabolites as precursors, such as actinomycin which is created from the primary
metabolite, tryptophan. Some sugars are metabolites, such as
fructose or glucose, which are both present in the metabolic pathways.
|
|
Class |
Example |
|
Alcohol |
Ethanol |
|
Amino acids |
Glutamic acid, aspartic acid |
|
Nucleotides |
5' guanylic acid |
|
Antioxidants |
Isoascorbic acid |
|
Organic acids |
Acetic acid, lactic acid |
|
Polyols |
Glycerol |
|
Vitamins |
B2 |
|
The metabolome forms a large network of metabolic
reactions, where outputs from one enzymatic chemical reaction are inputs to other chemical
reactions.
Metabolites from chemical compounds, whether inherent or pharmaceutical, are
formed as part of the natural biochemical process of degrading and eliminating
the compounds.[2] The rate of
degradation of a compound is an important determinant of the duration and
intensity of its action. Profiling metabolites of pharmaceutical compounds, drug metabolism, is an important part of drug discovery, leading to an understanding of
any undesirable side effects.
Examples of primary metabolites produced by industrial
microbiology:[1]
|
|
Leading with Quality, Performance and Cost
 Our partners, the
Pharmaffiliates Analytics and
Synthetics is a global leader for the integrated Contract Research
Organization (CRO).
Our partners, the
Pharmaffiliates Analytics and
Synthetics is a global leader for the integrated Contract Research
Organization (CRO).
With state of art research & development centers, advanced equipments, modern
facilities and well blending and highly experienced competent scientists and
experts and dynamic team of associates, Pharmaffiliates, since 2001, has been
focusing mainly on synthetic, analytical researches, NDDS and regulatory
consulting and certified processes to pump bio-pharmaceutical development
pipeline with highest quality, efficiency and cost advantages !
We regularly prepare stock or synthesis by request the following branches of
materials:
- Pharmaceutical Reference Standards
- Impurities & Metabolites Standards
- Pharmaceutical Material Standards
- Green Standards
- Food And Beverage Standards
- Agro-Chemical Standards Environmental Standards
Our Synthetic Service includes most categories of
customized compounds :
- Deuterium Compounds
- Non Pharmaceutical Standards
- Phyto-Chemical Standards
- Petrochemical Standards
- Pesticides Standards
- Food and Beverage Standards
- Environmental Standards
- Agro-Chemical Standards
Our team puts great emphasis on first-class quality. We
feel obliged to satisfy the highest demands, one of the keys to the success of
Pharmaffiliates.
Pharmaceutical Reference Standards

Green Standards
Food And Beverage Standards
Agro-Chemical Standards
Environmental Standards
List of Impurities & Metabolites
|
Product
Code
|
Product/Chemical
Name
|
Product Group/C ategory
|
Synonyms
|
Unit Size
|
Storage Condition s
|
CAS
No.
|
Molecular
Weight
|
Molecular
Formula
|
Other
Details
|
Other
Details
1
|
|
PA 01
01000
|
Abacavir
|
API
|
(1S,4R)-4-[2-Amino-6-
(cyclopropylamino)-9H-purin-9-yl]-2-
cyclopentene-1-methanol;
|
25MG,50MG
|
Refrigerator
|
136470-78-5
|
286.33
|
C14H18N6O
|
(1S-cis)-4-[2-Amino-6-
(cyclopropylamino)-9H-purin-9-yl]-
2-cyclopentene-1-methanol;
1592U89;
ABC;
|
Abacavir
- API
|
|
PA 01
01510
|
Descyclopropyl Abacavir
|
IH
|
(1S,4R)-4-(2,6-Diamino-9H-purin-9-yl)-2-
cyclopentene-1-methanol;
|
25MG,50MG
|
Freezer
|
124752-25-6
|
246.27
|
C11H14N6O
|
Abacavir Related Compound A;
|
Abacavir
- In house impurity
|
|
PA 01
01520
|
(1S,4R)-4-(2-Amino-6-chloro-9H-purin-9-
yl)-2-cyclopentene-1-methanol Hydrochloride
|
IH
|
(1S-cis)-4-(2-Amino-6-chloro-9H-purin-9-
yl)-2-cyclopentene-1-methanol Monohydrochloride;
|
25MG,50MG
|
Refrigerator, Under Inert Atmosphere
|
172015-79-1
|
302.16
|
C11H13Cl2N5O
|
Abacavir Related Compound C;
|
Abacavir
- In house impurity
|
|
PA 01
01530
|
trans-Abacavir Hydrochloride
|
IH
|
(1R,4R)-rel-4-[2-Amino-6-
(cyclopropylamino)-9H-purin-9-yl]-2-
cyclopentene-1-methanol Hydrochloride;
|
25MG,50MG
|
NA
|
267668-71-3
|
322.79
|
C14H19ClN6O
|
NA
|
Abacavir
- In house impurity
|
|
PA 01
01540
|
Abacavir Sulfate
|
IH
|
(1S,4R)-4-[2-Amino-6-
(cyclopropylamino)-9H-purin-9-yl]-2-
cyclopentene-1-methanol Sulfate; Ziagen;
|
25MG,50MG
|
Freezer
|
188062-50-2
|
670.47
|
C28H38N12O6S
|
NA
|
Abacavir
- In house impurity
|
|
PA 01
01550
|
Abacavir Carboxylate
|
IH
|
(1S,4R)-4-[2-Amino-6-
(cyclopropylamino)-9H-purin-9-yl]-2-
cyclopentene-1-carboxylic Acid;
2269W;
|
25MG,50MG
|
Hygroscopic, Freezer,
Under Inert Atmosphere
|
384380-52-3
|
300.32
|
C14H16N6O2
|
NA
|
Abacavir
- In house impurity
|
|
PA 01
01560
|
Abacavir 5’-β-D-Glucuronide
|
IH
|
[(1S,4R)-4-[2-amino-6-
(cyclopropylamino)-9H-purin-9-yl]-2-
cyclopenten-1-yl]methyl-β-D-
Glucopyranosiduronic
Acid;
Abacavir
5’-
Glucuronide;
|
25MG,50MG
|
Freezer,
Under
Inert
Atmosphere
|
384329-76-4
|
462.46
|
C20H26N6O7
|
NA
|
Abacavir
- In house impurity
|
|
PA 01
01570
|
Abacavir 5’-Phosphate
|
IH
|
(1S,4R)-
4-[2-Amino-6-
(cyclopropylamino)-9H-purin-9-yl]-2-
cyclopentene-1-methanol Dihydrogen Phosphate
(Ester);
|
25MG,50MG
|
Freezer
|
136470-77-4
|
366.31
|
C14H19N6O4P
|
(1S-cis)-Abacavir
Monophosphate;
|
Abacavir
- In house impurity
|
P1 - P977 - List_Impurities & Metabolites.pdf
|
|